Q&A: Pharmaceutical executive Dr. Antonio Grillo-Lopez talks reopening and vaccines
The United States government has been distributing vaccines for COVID-19 to the American people as part of the effort to stop the spread of the virus.
Aran Sonnad-Joshi
March 12, 2021
Dr. Antonio Grillo-Lopez is the former Chief Medical Officer of IDEC Pharmaceuticals where he led the clinical and regulatory development of Rituxan, the number one anti-cancer drug in the world. He also chaired the Neoplastic and Autoimmune Diseases Research Institute, which researches the acceleration of drug development and regulatory approval processes. Dr. Grillo-Lopez has also been an advisor to the National Cancer Institute and has served on the board of many pharmaceutical companies. Dr. Grillo Lopez has authored more than 400 publications and was an Associate Professor at the University of Michigan Medical School. During the pandemic, he has given numerous lectures and presentations about the vaccines themselves, distribution, and governmental approval.

Do you think schools should reopen now?
“I’m not sure that this is the time to go back. I don’t think that this is yet the time to fully open up schools, colleges, universities. We need to wait a little bit longer until we have more of the population immunized or have gotten the vaccines. Then it would be safer.
The other thing that’s happening at the same time as the vaccines are developed, is that we are also developing treatments in terms of antibodies, for example, or antivirals. That will not immunize you, but if you get the disease, you can be treated, and perhaps you’ll get just mild disease and not necessarily moderate or severe disease. So, to have a little bit more time for those to be more fully developed I think would be important.”
Do you think teachers should be higher on the list to receive the vaccine?
“Yeah, I think so because it’s a question of if you are infected how many, how many people are exposed? . . . So if you’re a teacher and you have 30-35 students in your classroom, plus you’re working with a number of other teachers and you’ll get together for lunch and so on, it’s likely that more than one will be infected. And it’s likely that more than one classroom will be exposed to the virus.”
What advice do you have for people who are going back to school?
“The measures to take are the simple measures. Wear a mask. If you can tolerate a double mask, do a double mask. Keep your distance. Wash your hands frequently. It’s amazing how those very simple measures go a long way.
And the same applies in school, you know, I’m sure that in school they’re going to sit you six feet apart from your other classmates and take all of those measures. Your teacher should not be speaking to you that close. She should keep her distance also, more than six feet for the teacher.
Spring and summer [are] coming up pretty soon, so open up your windows, make sure you have good ventilation. It would be ideal if schools had ventilation that has HEPA filters built-in, but I’m sure that most heating and ventilation systems in schools don’t have HEPA filters…. But, schools and other places where a lot of people congregate, theater, cinemas and so on, are going to have to do that because you’re sitting there for two/three hours, and you are, sometimes even recently breathing the same air. Ideally, you should be exhausting and bringing in fresh air, but, you know, that’s more expensive.
So it depends on what kind of ventilation system you have, but certainly not stay in the same classroom for three hours but take a break, go outside, move around, and don’t get close to anyone. And boys are gonna have to keep away from the girls for a while. I know that’s tough but hey.”
Will we achieve herd immunity and if so, when would that be?
Herd immunity occurs when a large enough percentage has become immune to the disease (either by getting the disease or receiving the vaccine) to stop the spread.
“I’m not optimistic about that because, as the disease has developed, initially it was said that 60 percent of the population needed to be immune in order to have herd immunity. And they’ve upgraded that to 70, and now they’re saying we are going to need 80 percent, at least, to have herd immunity.
It’s going to be very tough, it’s going to be very hard to get 80 percent immunity anytime soon. Certainly not a year from now even, I would think. In the US, for a number of reasons.
First, there’s a lot of people who are against the vaccines and they will just not listen to anything you can say. Their minds are made up: ‘We’re not going to take this stuff into our bodies.’ You know, you cannot convince them. And there are studies that have shown that. . . a year ago at the beginning of the pandemic, a survey, ‘Would you accept the vaccine? Yes or No’ showed that there was 70 percent acceptability. That has gone down, and it’s now around 60 percent acceptability. That’s the latest that I have seen. So, at that rate, you’re not going to get herd immunity.”
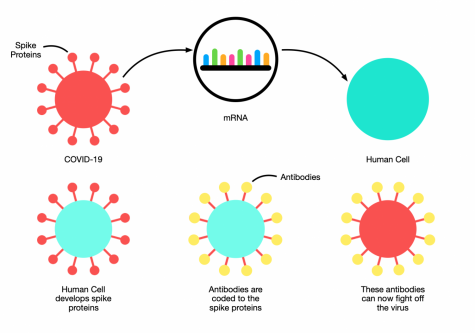
How do vaccines work to provide immunity?
“Well, the first two authorized, they aren’t approved, they’re authorized, in the US, Pfizer and Moderna are mRNA based. They are packaged so that RNA is delivered to your cells, your cells then are triggered by that RNA to produce spike protein, and the spike protein is manifested on the surface of your cells or even goes into the blood. Your body, your immune system, recognizes those spike proteins as foreign, reacts against them and you develop antibodies, T cells, memory B cells, memory T cells. That gives you immunity. The next time that your immune system sees a spike protein, it will attack that spike protein, and it will do away with the virus. You’re primed by the vaccine. You are immune. . . With AstraZeneca it’s chimp adenovirus that is used to deliver the spike protein. With [Johnson & Johnson] . . . I think it’s also an adenovirus.”
What are the potential side effects of the vaccine, and how frequent are they?
“In the very large studies by Pfizer, 40,000 [or] 44,000 people, and Moderna, 30,000+ about 60 percent or so did have some type of reaction, and it was usually fever, headache, muscle aches, chills, things of that nature, and locally, the injection site, redness, swelling, pain, inflammation. And, for example, in the Moderna studies, it mostly went away in two days. So it was in some cases severe but that was rare. In most cases, it was mild to moderate, the reactions.
I have personally seen some people who got no reactions at all, as was expected, but I’ve also seen some people with severe, severe headache, not responding even to not to aspirin or Tylenol not even to Tramadol, and having to consider going on to codeine, so severe the headache was and lasting several days. And also chills, very severe shaking, chills, not necessarily with a fever: sometimes with a fever, sometimes not. Things that those chills will make you achy all over because your muscles will be hurting after the chills.”
How does a vaccine get authorized, and what is the difference between the Emergency Use Authorization and regular approval?
The Emergency Use Authorization is a process that the Food and Drug Administration can use to temporarily allow the use of a drug in a state of emergency.
“Well, the EUA approval is not that frequent. Usually, when a pharmaceutical company has a product, they want to get it approved. Not necessarily authorized, they want to get it approved because full approval gets that product into the market.
And then the pharmaceutical company can get back the money that they have invested in developing that product and they can also make some profit that goes to the shareholders. In terms of value-added to the stock price and so on.
But, importantly, it’s not just about that. It’s about making that product available to the general public so that if your physician prescribes it, you can go to any drugstore and get it. So that’s what they usually go for full approval.
The emergency use authorization the EUA gets you authorized, earlier. [It] makes the product available earlier. However, the system is such that there is not as full a review of all of the data, as you would have for an approval, and it’s faster. So, it’s useful if you want to get there faster, which is the situation right now. There are other mechanisms that could be used.
There’s one that I have used for example which is called an accelerated approval. Which, as the word says gets you there earlier, but it’s the equivalent of a full approval. One difference that’s important between the two is that with a EUA there are a lot of restrictions that the FDA can impose as to who can receive the product, who can deliver that product to patients. So the FDA has more control, has more requirements, has imposed restrictions, because it’s not a full approval, and they’re concerned that it might not be used correctly.”
What mistakes were made during vaccine development and distribution?
“I felt that the vaccine should have been approved back in June/July of 2020. And then, more people could have been immunized earlier and fewer people would have died in the six months that it took to get the EUA. The reason for that was that at that time, Pfizer, Moderna, and AstraZeneca had published their initial results, which were not the huge 30 and 40,000 patient studies but added up between the three of them they have probably 1500 patients or so. More important than the number of patients was the fact that they showed that people who were immunized people who get the vaccines, developed very significant amounts of antibodies. They were immune. The effect was good, there were T cells produced, so these people were effectively immunized against Covid.
So, why go to such huge numbers? (Which are unprecedented by the way because there is a review published I think about a year ago, of all of the studies that have been done recently in the last 10 years.) I think for vaccines, different vaccines, there’s about 28 vaccines approved nowadays, and the ones that they reviewed had submitted, not more than 5000 subjects per vaccine submitted to the FDA for approval. So why, if that is the precedent, 5000 or so. . . why go now to 30 40,000? To me, that was not warranted, and the price was that [in] the six months it took to conduct all of those stories and generate the data and get a EUA, people were dying during that time. And it was not a few; it was large numbers of people that died.”
How should we be better prepared for next time?
“I think that it’s very difficult to be prepared for something like this. Well, it’s new, de novo. Now that we have the experience, we’ll know what to do the next time. And by the way, that next time is now. Because as you know, the virus is changing, which was to be expected. That should not be news to anyone. The media is making a big deal of ‘The virus is here to stay!”But for heaven’s sakes, did anyone think it was really going to go away entirely? No. The virus is here and there will be mutations, and there will be variants. And I assure you that. Moderna, Pfizer and other companies are already working on the new and improved vaccine to take care of the variants and so on because that now becomes, I don’t want to minimize the importance of this but it’s sort of routine now, just like influenza, where every year you have to produce a new vaccine.”
Will life return to normal and if so, when would that happen?
“Are we back to normal with influenza? Probably not. We have a lot of people who die from influenza every year. So from that point of view, we’re not back to pre-influenza normal where no one died from that disease. I think we are going to have, unfortunately, deaths from Covid even though we develop newer vaccines, even though we immunize more people. So we’re not going to get back to a normal from that point of view in the next few years. Who knows what’ll happen after that?
Normal in terms of going back to school, going back to work, opening bars, restaurants, and so on? Yeah, I think that more people get immunized that will happen. Life has to go on, I mean we cannot be under lockdown for another year. It’s been a real hit to our economy.”